
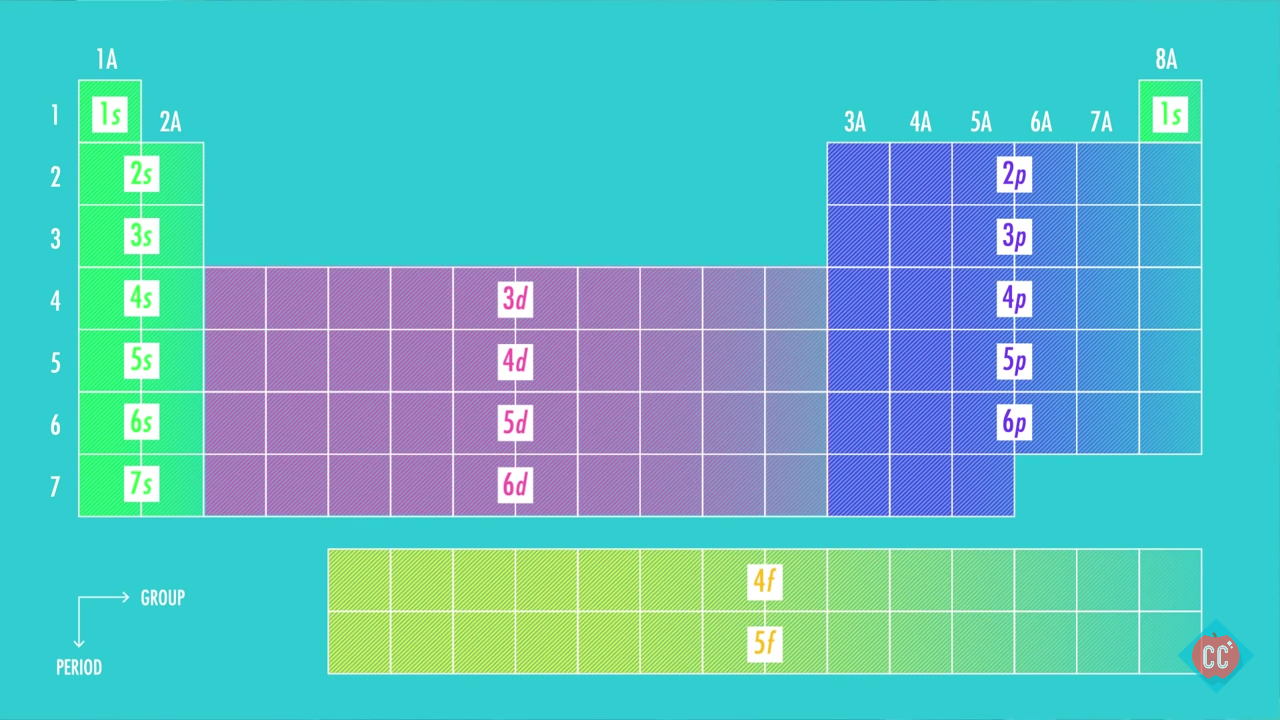
Thus, the two electrons in the carbon 2 p orbitals have identical n, l, and m s quantum numbers and differ in their m l quantum number (in accord with the Pauli exclusion principle). The ground state electronic configuration of Krypton is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. The orbitals are filled as described by Hund’s rule: the lowest-energy configuration for an atom with electrons within a set of degenerate orbitals is that having the maximum number of unpaired electrons. Consider the element with the electron configuration (Kr)5s24d105p5.
Electron configuration for krypton full#
We now have a choice of filling one of the 2 p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but degenerate, p orbitals. Full electron configuration of krypton: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. The electron configuration of strontium ion (Sr 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. The remaining two electrons occupy the 2 p subshell. The strontium atom donates two electrons of the last shell to form bonds and turns into a strontium ion (Sr 2+ ). Kr A: Electronic configuration is a distribution of electrons of a atoms in a atomic or moleculer orbital. Four of them fill the 1 s and 2 s orbitals. A: Given :- electron configuration of an element Xe6s14f145d9 To identify :- class of that element Q: Complete the electron configuration for I. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling.Ĭarbon (atomic number 6) has six electrons. There are three degenerate 2 p orbitals ( m l = −1, 0, +1) and the electron can occupy any one of these p orbitals. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2 p orbital. The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Its electron configuration is shown below.
The fourth electron fills the remaining space in the 2 s orbital.Īn atom of boron (atomic number 5) contains five electrons. As such, it has a stable octet in its valence shell and is extremetly unreactive. Thus, the electron configuration and orbital diagram of lithium are:Īn atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. Bromine (Group 7A) has the electron configuration Ar 3d104s24p4 to form Br, it gains one electron to achieve the electron configuration of krypton.


 0 kommentar(er)
0 kommentar(er)
